According to Stanford University's findings, the aluminum-ion battery consists of two electrodes, one negatively charged anode made of aluminum and a positively charged cathode. Scientists at Stanford University accidently discovered graphite as the simple solution. For the experimental battery, the Stanford team placed the aluminium anode and graphite cathode, along with an ionic liquid electrolyte, inside a flexible polymer-coated pouch. "The electrolyte is basically a salt that's liquid at room temperature, so it's very safe," said Stanford graduate student Ming Gong.
The aluminum-ion battery could eventually replace many of the lithium-ion and alkaline batteries used in many smartphones today. The aluminium-ion battery was able to withstand more than 7,500 cycles without any capacity loss whereas lithium-ion batteries generally last about 1,000 cycles. Aluminum batteries could also eventually be used for storing renewable energy on electrical grids, which need batteries with a long life cycle to both store and release energy.
CONSUMER
Stanford University develops aluminium-ion battery
It can fully charge your smartphone within a minute
April 6, 2015
Lithium-ion batteries have long been the only option in the modern world – they replaced the heavier single-use alkaline batteries used in everything from wristwatches to aeroplanes. Unfortunately, both of these are already struggling to keep up with our ever-increasing energy needs.
Last year, Tech Crunch reported that the global smartphone audience is forecast to reach 6.1 billion users by 2020. As smartphones and other technology increasingly develop, the demand for longer-lasting batteries will continue to increase.
Researchers have been interested in developing a commercially viable aluminum-ion battery for decades, but efforts have been largely unsuccessful. Fortunately, researchers at Stanford University have developed an aluminum-ion battery prototype! Crucially, aluminum-ion technology is an environmentally friendly alternative to disposable alkaline batteries.
Researchers have been interested in developing a commercially viable aluminum-ion battery for decades, but efforts have been largely unsuccessful. Fortunately, researchers at Stanford University have developed an aluminum-ion battery prototype! Crucially, aluminum-ion technology is an environmentally friendly alternative to disposable alkaline batteries.
The all new aluminium-ion battery is truly high performance, long lasting, inexpensive and has a high storage capacity. Researchers say that the new technology offers a safe alternative to many commercial batteries in wide use today. Best yet, the battery completely charges in just over a minute. The aluminum battery is also flexible so it can be used in electronic devices that can fold and bend, an increasing trend in modern technologies.
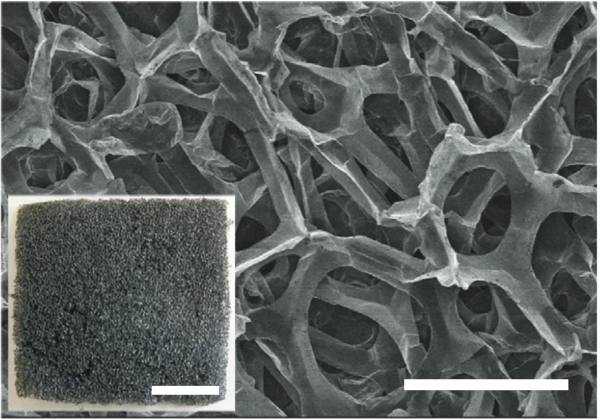
Stanford's aluminum-ion battery uses a unique three-dimensional graphite foam to speed up the movement of ions inside the battery, and unlock its unprecedented charging and discharging times. Photo: Nature.com
The new aluminium-ion battery offers a safer alternative to its predecessors, being fire proof. "We have developed a rechargeable aluminum battery that may replace existing storage devices, such as alkaline batteries, which are bad for the environment, and lithium-ion batteries, which occasionally burst into flames," said Stanford University chemistry professor Hongjie Dai, the lead researcher of the project.