Such precious stones as ruby, sapphire, aquamarine and emerald are also aluminium minerals. The first two are corundum, i.e. aluminium oxide (Al2O3) in crystal form. It's naturally transparent and in terms of strength it's only second to diamonds. Sapphire is used in bullet proof glass, aeroplane windows, scratch resistant smartphone screens. In the meantime one of the less precious corundum minerals, emery, is used as an abrasive, for example in sand cloth.
WHAT IS ALUMINIUM?
WHAT IS ALUMINIUM?
Light, durable and functional: these are the qualities that make aluminium one of the key engineering materials of our time. We can find aluminium in the homes we live in, in the automobiles we drive, in the trains and aeroplanes that take us across long distances, in themobile phones and computers we use on a daily basis, in the shelves inside our fridges and in modern interior designs, but a mere 200 years ago very little was known about this metal.
Ease Comes through Hard Work
Ivan Aivazovsky
a famous Russian artist
Ivan Aivazovsky
a famous Russian artist

Aluminium is a silvery-white metal, the 13 element in the periodic table. One surprising fact about aluminium is that it's the most widespread metal on Earth, making up more than 8% of the Earth's core mass. It's also the third most common chemical element on our planet after oxygen and silicon.
At the same time, because it easily binds with other elements, pure aluminium does not occur in nature. This is the reason that people learned about it relatively recently. Formally aluminium was produced for the first time in 1824 and it took people another fifty years to learn to produce it on an industrial scale.
The most common form of aluminium found in nature is aluminium sulphates. These are minerals that combine two sulphuric acids: one based on an alkaline metal (lithium, sodium, potassium rubidium or caesium) and one based on a metal from the third group of the periodic table, primarily aluminium.
Aluminium sulphates are used to this day to clean water, for cooking, in medicine, in cosmetology, in the chemical industry and in other sectors. By the way, aluminium got its name from aluminium sulphates which in Latin were called alumen.
At the same time, because it easily binds with other elements, pure aluminium does not occur in nature. This is the reason that people learned about it relatively recently. Formally aluminium was produced for the first time in 1824 and it took people another fifty years to learn to produce it on an industrial scale.
The most common form of aluminium found in nature is aluminium sulphates. These are minerals that combine two sulphuric acids: one based on an alkaline metal (lithium, sodium, potassium rubidium or caesium) and one based on a metal from the third group of the periodic table, primarily aluminium.
Aluminium sulphates are used to this day to clean water, for cooking, in medicine, in cosmetology, in the chemical industry and in other sectors. By the way, aluminium got its name from aluminium sulphates which in Latin were called alumen.

Corundum
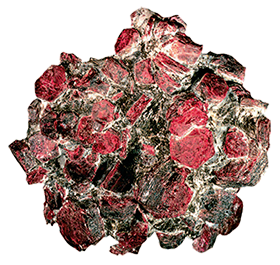
Today we know about almost 300 various aluminium compounds and minerals containing aluminium, from feldspar, a key source mineral on Earth, to ruby, sapphire and emerald, which are far less common.

Humphry Davy. The British physicist and chemist Sir Humphry Davy was the first to obtain a new chemical element using electrolysis: he was able to obtain boron from boric acid. He went on to use electrolysis to isolate six more previously unknown metals: potassium, sodium, barium, calcium, magnesium and strontium. It was Davy who proved the existence of aluminium, the metal found in alumina, and gave it its name.
But regardless of how common aluminium may be, it may have remained hidden forever if it hadn't been for electricity. The discovery of aluminium was made possible when scientists were able to use electricity to break down chemical compounds into their elements. In the 19 century the Danish physicist Christian Oersted used electrolysis to obtain aluminium. Electrolysis or electrolytic reduction is the process that is used to produce aluminium today as well.
Another rather common mineral, bauxite, is used today as the primary raw material in aluminium production. Bauxite is a clay mineral comprising various modifications of aluminium hydroxide mixed with iron, silicon, titanium, sulphur, gallium, chromium, vanadium oxides, as well as sulphuric calcium, iron and magnesium carbonates. In other words, your typical bauxite contains almost half the periodic table. By the way, because of the texture of bauxite about a hundred years ago aluminium was often referred to rather poetically as silver obtained from clay. On the average 4-5 tonnes of bauxite are needed to produce 1 tonne of aluminium.
Another rather common mineral, bauxite, is used today as the primary raw material in aluminium production. Bauxite is a clay mineral comprising various modifications of aluminium hydroxide mixed with iron, silicon, titanium, sulphur, gallium, chromium, vanadium oxides, as well as sulphuric calcium, iron and magnesium carbonates. In other words, your typical bauxite contains almost half the periodic table. By the way, because of the texture of bauxite about a hundred years ago aluminium was often referred to rather poetically as silver obtained from clay. On the average 4-5 tonnes of bauxite are needed to produce 1 tonne of aluminium.
Bauxites
Bauxites were discovered in 1821 by geologist Pierre Berthier in Southern France. The new minerals were named after the area they were discovered in: Les Baux. About 90% of the global supply of bauxites is found in tropical and subtropical areas such as Guinea, Australia, Vietnam, Brazil, India and Jamaica.

In the first stage of aluminium production bauxite is processed into alumina, or aluminium oxide Al2O3. Alumina looks like white powder and it is then processed into aluminium at aluminium smelters using electrolytic reduction.
Aluminium production requires huge amounts of electricity, about 15 MWH per tonne of output. That's approximately as much as a 100-apartment block consumes in a month. So the best site for an aluminium smelter is next to a powerful, preferably renewable, energy source. Hydroelectric power plants are the best option as they are the most powerful 'green' energy sources available today.
Aluminium production requires huge amounts of electricity, about 15 MWH per tonne of output. That's approximately as much as a 100-apartment block consumes in a month. So the best site for an aluminium smelter is next to a powerful, preferably renewable, energy source. Hydroelectric power plants are the best option as they are the most powerful 'green' energy sources available today.
Properties of aluminium
Aluminium offers a rare combination of valuable properties. It is one of the lightest metals in the world: it's almost three times lighter than iron but it's also very strong, extremely flexible and corrosion resistant because its surface is always covered in an extremely thin and yet very strong layer of oxide film. It doesn't magnetise, it's a great electricity conductor and forms alloys with practically all other metals.

Lightweight
Three times lighter than iron

Durable
Almost as much as steel

Ductile
Easy in processing

Corrosion-resistant
Due to a thin surface layer of aluminium oxide
Aluminium can be easily processed using pressure both when it's hot and when it's cold. It can be rolled, pulled and stamped. Aluminium doesn't catch fire, it doesn't need special paint and unlike plastics it's not toxic. It's also very pliable so sheets just 4 microns thick can be made from it, as well as extra thin wire. The extra-thin foil that can be made from aluminium is three times thinner than a human hair. In addition, aluminium is more cost effective than other metals and materials.
Since aluminium easily forms compounds with other chemical elements, a huge variety of aluminium alloys have been developed. Even a very small amount of admixtures can drastically change the properties of the metal, making it possible to use it in new areas. For example, in ordinary life you can find aluminium mixed with silicon and magnesium literally on the road, i.e. in the aluminium alloy wheels, in the engines, chassis and other parts of modern automobiles. As for aluminium zinc alloy, chances are you might be holding it in your hands right now as it's this alloy that's widely used in the production of mobile phones and tablet PCs. In the meantime, scientists keep developing new aluminium alloys.
The modern construction, automotive, aviation, energy, food and other industries would be impossible without aluminium. In addition, aluminium has become a symbol of progress: all cutting edge devices and vehicles are made from aluminium. (link to the Uses section)
Since aluminium easily forms compounds with other chemical elements, a huge variety of aluminium alloys have been developed. Even a very small amount of admixtures can drastically change the properties of the metal, making it possible to use it in new areas. For example, in ordinary life you can find aluminium mixed with silicon and magnesium literally on the road, i.e. in the aluminium alloy wheels, in the engines, chassis and other parts of modern automobiles. As for aluminium zinc alloy, chances are you might be holding it in your hands right now as it's this alloy that's widely used in the production of mobile phones and tablet PCs. In the meantime, scientists keep developing new aluminium alloys.
The modern construction, automotive, aviation, energy, food and other industries would be impossible without aluminium. In addition, aluminium has become a symbol of progress: all cutting edge devices and vehicles are made from aluminium. (link to the Uses section)

Aluminium vs Copper
If all copper wire were replaced with aluminium-zirconium wire in a car, the weight of the vehicle would be 12 kg less

Aluminium supplies
The International Aluminium Institute (IAI) estimates there are currently 400 million tonnes of aluminium being used in infrastructure, transport and domestically
It would seem that the mix of qualities enumerated above would already be enough to make aluminium the top choice in industry, however, there is another property that is just as significant: aluminium can be reused over and over again. Both aluminium and its alloys can be melted down and reused without any detriment to its mechanical properties. Scientists have estimated that 1 kg of recycled aluminium cans can save up to 8 kg of bauxite, 4 kg of various fluorides and up to 15 KWH of electricity.
About 75% of aluminium produced in the time that the aluminium industry has existed is still in use today.
Photo: © Shutterstock and © Rusal.
About 75% of aluminium produced in the time that the aluminium industry has existed is still in use today.
Photo: © Shutterstock and © Rusal.